Quest for the right Drug
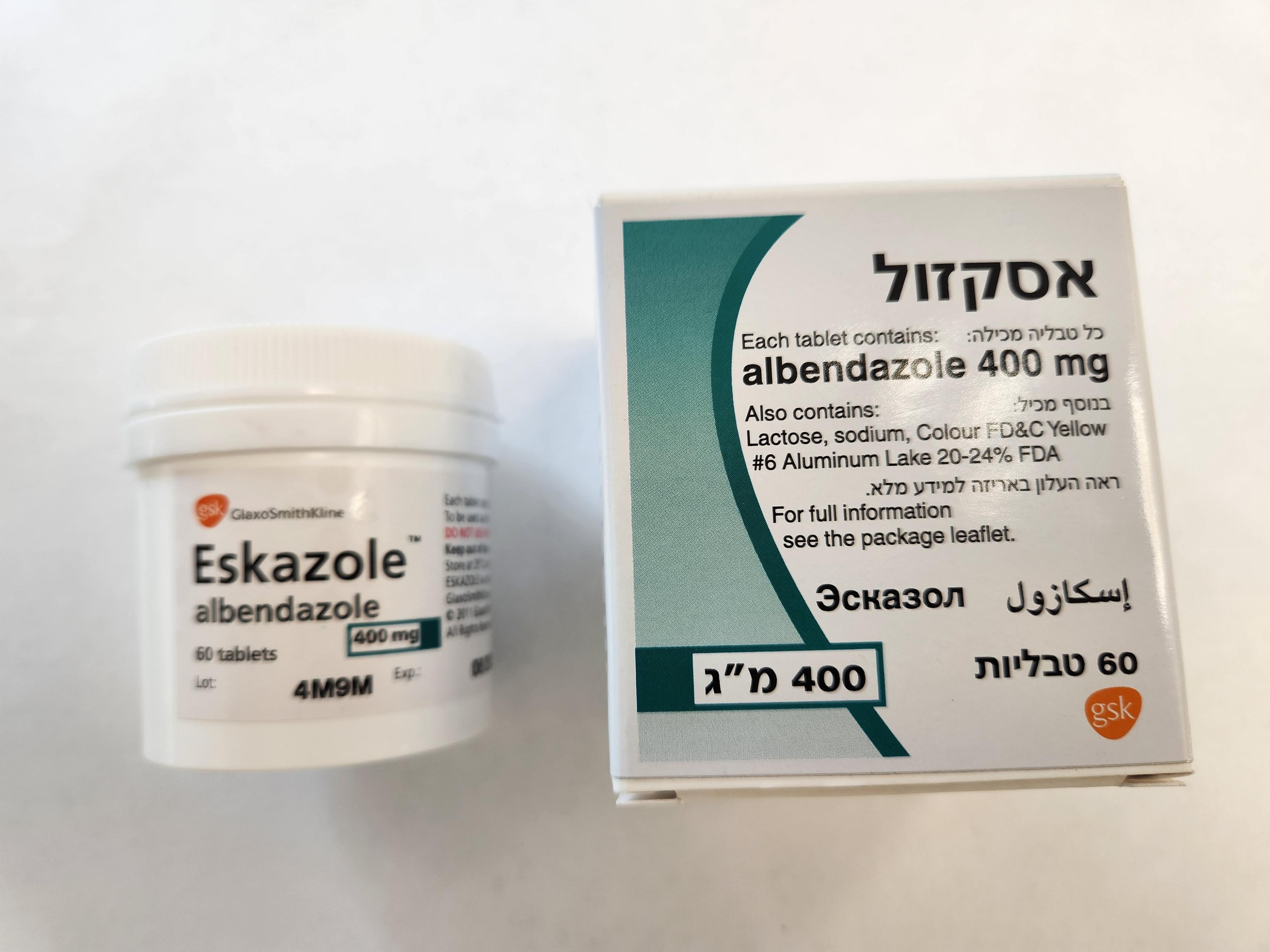
אסקזול ESKAZOLE (ALBENDAZOLE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : PER OS
צורת מינון:
טבליה : TABLETS
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Special Warning : אזהרת שימוש
4.4. Special warnings and precautions for use Treatment with albendazole has been associated with mild to moderate elevations of hepatic enzymes. Hepatic enzymes generally normalized after treatment discontinuation. Cases of hepatitis have been reported (see section 4.8). Therefore, hepatic function tests should be obtained before the start of each treatment cycle, and at least every two weeks during treatment. If hepatic enzymes are significantly increased (greater than twice the upper limit of normal), treatment should be discontinued. Treatment may be restarted when hepatic enzymes have returned to normal limits, but patients should be carefully monitored for recurrence. Patients with abnormal hepatic function test results prior to initiating treatment should be closely monitored due to the hepatotoxic potential of albendazole. Albendazole has been shown to cause bone marrow suppression and therefore, blood counts should be performed at the start of treatment and every two weeks during each 28-day cycle. Patients with hepatic disease, including hepatic echinococcosis, appear to be more susceptible to bone marrow suppression leading to pancytopenia, aplastic anemia, agranulocytosis and leukopenia, and therefore warrant closer monitoring of blood counts. Treatment with albendazole should be discontinued if clinically significant decreases in blood cell counts occur (see sections 4.2 and 4.8). In order to avoid administering albendazole during early pregnancy, women of child-bearing age should: - initiate treatment only after a negative pregnancy test. These tests should be repeated at least once before initiating the next cycle. - be advised to take effective contraceptive measures during and for one month after completion of treatment with albendazole for a systemic infection. Pre-existing neurocysticercosis may also be detected in patients treated with albendazole for other conditions, particularly in areas with high taenosis infection. Patients may experience neurological symptoms such as seizures, increased intracranial pressure and focal signs as a result of an inflammatory reaction caused by death of the parasite within the brain. Symptoms may occur soon after treatment, and the appropriate treatment with anticonvulsant drugs and steroids should be started immediately. In rare cases of retinal neurocysticercosis, the patient should be examined for retinal lesions before beginning treatment. If these lesions are observed, the benefit of the therapy should be weighed against the possibility of retinal damage. Warnings for excipients This medicinal product contains lactose. Patients with hereditary galactose intolerance, the Lapp lactase deficiency (deficiency observed in certain populations of Laponia) or glucose-galactose malabsorption should not take this medicine. This medicinal product may cause allergic reactions because it contains Colour FD&C Yellow #6 Aluminum Lake 20-24% FDA (E110) colorant.
Effects on Driving

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
עלון מידע לצרכן
03.05.18 - עלון לצרכן 27.02.20 - עלון לצרכן אנגלית 27.02.20 - עלון לצרכן עברית 27.02.20 - עלון לצרכן ערבית 03.01.23 - עלון לצרכן עברית 05.06.23 - עלון לצרכן אנגלית 05.06.23 - עלון לצרכן עברית 05.06.23 - עלון לצרכן ערבית 10.10.24 - עלון לצרכן עברית 30.11.11 - החמרה לעלון 19.04.17 - החמרה לעלוןלתרופה במאגר משרד הבריאות
אסקזול