Quest for the right Drug
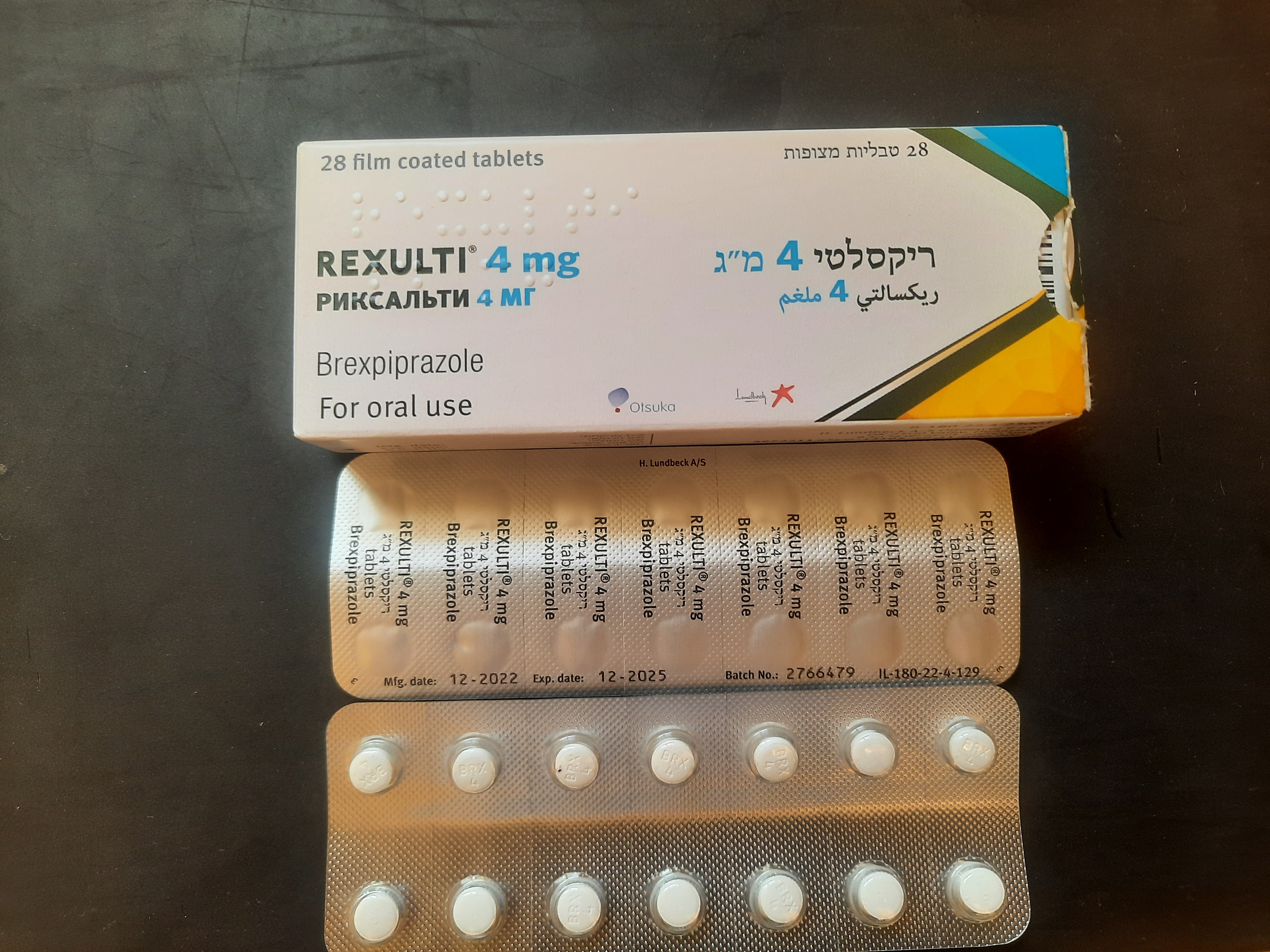
ריקסלטי 4 מ"ג REXULTI ® 4 MG (BREXPIPRAZOLE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : PER OS
צורת מינון:
טבליות מצופות פילם : FILM COATED TABLETS
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
6 ADVERSE REACTIONS The following adverse reactions are discussed in more detail in other sections of the labeling: • Increased Mortality in Elderly Patients with Dementia-Related Psychosis [see Boxed Warning, Warnings and Precautions (5.1)] • Suicidal Thoughts and Behaviors in Adolescents and Young Adults [see Boxed Warning, Warnings and Precautions (5.2)] • Cerebrovascular Adverse Reactions Including Stroke in Elderly Patients with Dementia-Related Psychosis [see Warnings and Precautions (5.3)] • Neuroleptic Malignant Syndrome (NMS) [see Warnings and Precautions (5.4)] • Tardive Dyskinesia [see Warnings and Precautions (5.5)] • Metabolic Changes [see Warnings and Precautions (5.6)] • Pathological Gambling and Other Compulsive Behaviors [see Warnings and Precautions (5.7)] • Leukopenia, Neutropenia, and Agranulocytosis [see Warnings and Precautions (5.8)] • Orthostatic Hypotension and Syncope [see Warnings and Precautions (5.9)] • Falls [see Warnings and Precautions (5.10)] • Seizures [see Warnings and Precautions (5.11)] • Body Temperature Dysregulation [see Warnings and Precautions (5.12)] • Dysphagia [see Warnings and Precautions (5.13)] • Potential for Cognitive and Motor Impairment [see Warnings and Precautions (5.14)] 6.1 Clinical Trials Experience Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice. Adjunctive Treatment in Major Depressive Disorder (MDD) The safety of REXULTI was evaluated in 1054 adult patients (18 to 65 years of age) diagnosed with MDD who participated in two 6-week, placebo-controlled, fixed-dose clinical studies in patients with major depressive disorder in which REXULTI was administered at doses of 1 mg to 3 mg daily as adjunctive treatment to continued antidepressant therapy; patients in the placebo group continued to receive antidepressant therapy [see Clinical Studies (14.1)]. Adverse Reactions Reported as Reasons for Discontinuation of Treatment A total of 3% (17/643) of REXULTI-treated patients and 1% (3/411) of placebo-treated patients discontinued due to adverse reactions. Adverse Reactions in REXULTI Studies for Adjunctive MDD in Adults Adverse reactions associated with the adjunctive use of REXULTI (incidence of 2% or greater and adjunctive REXULTI incidence greater than adjunctive placebo) that occurred during acute therapy (up to 6-weeks in patients with MDD) are shown in Table 8. Table 8: Adverse Reactions in ≥2% of REXULTI-Treated Patients and Greater than Placebo in Pooled 6-Week Placebo-Controlled, Fixed-Dose Adjunctive MDD Studies in Adults (Study 1 and Study 2) REXULTI Placebo All 1 mg/day 2 mg/day 3 mg/day (N=411) REXULTI % (N=226) (N=188) (N=229) % % % (N=643) % Gastrointestinal Disorders Constipation 1 3 2 1 2 General Disorders and Administration Site Conditions Fatigue 2 3 2 5 3 Infections and Infestations Nasopharyngitis 2 7 1 3 4 Investigations Weight Increased 2 7 8 6 7 Blood Cortisol 2 Decreased 1 4 0 3 Metabolism and Nutrition Increased Appetite 2 3 3 2 3 Nervous System Disorders Akathisia 2 4 7 14 9 Headache 6 9 4 6 7 Somnolence 0.5 4 4 6 5 Tremor 2 4 2 5 4 Dizziness 1 1 5 2 3 Psychiatric Disorders Anxiety 1 2 4 4 3 Restlessness 0 2 3 4 3 Dose-Related Adverse Reactions in the Adjunctive MDD Studies In Studies 1 and 2, among the adverse reactions that occurred at ≥2% incidence in the patients treated with REXULTI plus ADT, the incidences of akathisia and restlessness increased with increases in dose. Schizophrenia Adults The safety of REXULTI was evaluated in 852 adult patients (18 to 65 years of age) diagnosed with schizophrenia who participated in two 6-week placebo-controlled, fixed-dose clinical studies in which REXULTI was administered at daily doses of 1 mg, 2 mg and 4 mg [see Clinical Studies (14.2)]. Adverse Reactions Occurring at an Incidence of 2% or More in Patients Treated with REXULTI for Schizophrenia Adverse reactions associated with REXULTI (incidence of 2% or greater and REXULTI incidence greater than placebo) during short-term (up to 6-weeks) studies in adult patients with schizophrenia are shown in Table 9. Table 9: Adverse Reactions in ≥2% of REXULTI-Treated Patients and Greater than Placebo in Pooled 6-Week Placebo-Controlled, Fixed-Dose Schizophrenia Studies in Adult Patients (Study 3 and Study 4) REXULTI Placebo ALL (N=368) 1 mg/day 2 mg/day 4 mg/day REXULTI % (N=120) (N=368) (N=364) % % % (N=852) % Gastrointestinal Disorders Dyspepsia 2 6 2 3 3 Diarrhea 2 1 3 3 3 Investigations Weight 2 3 4 4 4 Increased Blood 1 4 2 2 2 Creatinine Phosphokinase Increased Nervous System Disorders Akathisia 5 4 5 7 6 Tremor 1 2 2 3 3 Sedation 1 2 2 3 2 Agitation Associated with Dementia Due to Alzheimer’s Disease The safety of REXULTI was evaluated in 503 patients (51 to 90 years of age), with a probable diagnosis of agitation associated with dementia due to Alzheimer’s disease, who participated in two 12-week placebo-controlled, fixed-dose clinical studies in which REXULTI was administered at daily doses of 2 mg to 3 mg [see Clinical Studies (14.3)]. Discontinuation of Treatment Due to Adverse Reactions In two 12-week placebo-controlled, fixed-dose, clinical studies, a total of 5.6% (28/503) of patients treated with REXULTI and 4.8% (12/251) of patients treated with placebo discontinued due to adverse reactions. Adverse Reactions Occurring at an Incidence of 2% or More in Patients Treated with REXULTI for Agitation Associated with Dementia Due to Alzheimer’s Disease Adverse reactions associated with REXULTI (incidence ≥2% and greater than placebo) during the 12-week fixed-dose clinical studies in geriatric patients for treatment of agitation associated with dementia due to Alzheimer’s disease are shown in Table 10. Table 10 Adverse Reactions in ≥2% of REXULTI-Treated Patients and Greater than Placebo in Pooled 12-Week Placebo-Controlled, Fixed-Dose Agitation Associated with Dementia due to Alzheimer’s Disease Studies (Study 6 and Study 7) REXULTI ALL Placebo 1 mg/day* 2 mg/day 3 mg/day REXULTI (N=251) (N=137) (N=213) (N=153) (N=503) % % % % % Infections and Infestations Nasopharyngitis 2 4 2 3 3 Urinary Tract Infection 1 2 3 3 3 Nervous System Disorders Dizziness† 2 1 5 3 3 Headache 8 9 9 7 8 ‡ Somnolence 1 2 3 4 3 Psychiatric Disorders Insomnia§ 3 5 5 2 4 *1 mg once day REXULTI dosage is not a recommended dosage for the treatment of agitation associated with dementia due to Alzheimer’s disease [see Dosage and Administration (2.4)]. †Dizziness and Vertigo are grouped to Dizziness ‡Sedation and somnolence are group to somnolence. §Initial insomnia and insomnia are grouped to insomnia Extrapyramidal Symptoms Adjunctive Treatment of Major Depressive Disorder The incidence of reported extrapyramidal symptoms (EPS)-related adverse reactions, excluding akathisia, was 6% for REXULTI plus ADT-treated patients versus 3% for placebo plus ADT-treated patients. The incidence of akathisia events for REXULTI plus ADT-treated patients was 9% versus 2% for placebo plus ADT-treated patients. In the 6-week placebo-controlled MDD studies, data was objectively collected on the Simpson - Angus Rating Scale (SAS) for EPS, the Barnes Akathisia Rating Scale (BARS) for akathisia and the Abnormal Involuntary Movement Score (AIMS) for dyskinesia. The mean change from baseline at last visit for REXULTI plus ADT-treated patients for the SAS, BARS and AIMS was comparable to placebo - treated patients. The percentage of patients who shifted from normal to abnormal was greater in REXULTI plus ADT-treated patients versus placebo plus ADT-treated patients for the BARS (4% versus 0.6%) and the SAS (4% versus 3%). Schizophrenia The incidence of reported EPS-related adverse reactions, excluding akathisia, was 5% for REXULTI-treated patients versus 4% for placebo-treated patients. The incidence of akathisia events for REXULTI-treated patients was 6% versus 5% for placebo-treated patients. In the 6-week placebo-controlled, fixed-dose schizophrenia studies in adults, data was objectively collected on the Simpson - Angus Rating Scale (SAS) for EPS, the Barnes Akathisia Rating Scale (BARS) for akathisia and the Abnormal Involuntary Movement Scale (AIMS) for dyskinesia. The mean change from baseline at last visit for REXULTI-treated patients for the SAS, BARS and AIMS was comparable to placebo-treated patients. The percentage of patients who shifted from normal to abnormal was greater in REXULTI-treated patients versus placebo for the BARS (2% versus 1%) and the SAS (7% versus 5%). Agitation Associated with Dementia Due to Alzheimer’s Disease The incidence of reported EPS-related adverse reactions, excluding akathisia, was 3% for REXULTI-treated patients versus 2% for placebo-treated patients. The incidence of akathisia events for REXULTI-treated patients was 1% versus 0% for placebo-treated patients. In the 12-week placebo-controlled, fixed-dose studies in agitation associated with dementia due to Alzheimer’s disease, data was objectively collected on the Simpson-Angus Rating Scale (SAS) for EPS, the Barnes Akathisia Rating Scale (BARS) for akathisia and the Abnormal Involuntary Movement Scale (AIMS) for dyskinesia. The mean change from baseline at last visit for REXULTI-treated patients for the SAS, BARS and AIMS was comparable to placebo-treated patients. The percentage of patients who shifted from normal to abnormal was greater in REXULTI-treated patients versus placebo for the SAS (6% versus 2%). Dystonia Symptoms of dystonia may occur in susceptible individuals during the first few days of treatment. Dystonic symptoms include: spasm of the neck muscles, sometimes progressing to tightness of the throat, swallowing difficulty, difficulty breathing, and/or protrusion of the tongue. While these symptoms can occur at low doses, they occur more frequently and with greater severity with high potency and at higher doses of first - generation antipsychotic drugs. An elevated risk of acute dystonia is observed in males and younger age groups. Other Adverse Reactions Observed during Clinical TrialEvaluation of REXULTI Other adverse reactions (≥1% frequency and greater than placebo) within the short-term, placebo-controlled trials in adult patients with MDD and schizophrenia are shown below. The following listing does not include adverse reactions: 1) already listed in previous tables or elsewhere in the labeling, 2) for which a drug cause was remote, 3) which were so general as to be uninformative, 4) which were not considered to have clinically significant implications, or 5) which occurred at a rate equal to or less than placebo. Eye Disorders: Vision Blurred Gastrointestinal Disorders: Nausea, Dry Mouth, Salivary Hypersecretion, Abdominal Pain, Flatulence Investigations: Blood Prolactin Increased Musculoskeletal and Connective Tissue Disorders: Myalgia Psychiatric Disorders: Abnormal Dreams Skin and Subcutaneous Tissue Disorders: Hyperhidrosis Pediatric Patients (13 to 17 years of age) In an on-going, 2 year, open-label study in pediatric patients 13 to 17 years of age with schizophrenia, in which safety was assessed in 194 patients of which 140 received REXULTI for at least 6 months. Adverse reactions reported in clinical studies for this age group were generally similar to those observed in adult patients. Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form https://sideeffects.health.gov.il/ 6.2 Postmarketing Experience The following adverse reaction has been identified during post-approval use of REXULTI. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. Nervous System disorders: Neuroleptic Malignant Syndrome

פרטי מסגרת הכללה בסל
הטיפול בתרופה יינתן למקרים האלה:א. מבוטח בגיר ומתבגר מגיל 13 ומעלה שהוא חולה סכיזופרניה, אשר עונה על אחד מהתנאים האלה: 1. פיתח תופעות לוואי לטיפול קודם ב-Aripiprazole; 2. הגיב חלקית לטיפול בתרופה אנטי פסיכוטית שניתנה לו כקו טיפול קודם, והוא מועמד לטיפול בתכשיר אנטי פסיכוטי מסוג D2 partial agonist; התחלת הטיפול בתרופה תהיה על פי הוראתו של רופא מומחה בפסיכיאטריה או בפסיכיאטריה של הילד והמתבגר, לפי העניין. לא יינתנו לחולה בו בזמן שתי תרופות או יותר ממשפחת התרופות האנטיפסיכוטיות האטיפיות.ב. טיפול אוגמנטציה בדיכאון מסוג major depressive disorder (MDD), עבור חולים שפיתחו תופעות לוואי ל-Aripiprazole. התחלת הטיפול בתרופה תהיה על פי הוראתו של רופא מומחה בפסיכיאטריה.
מסגרת הכללה בסל
התוויות הכלולות במסגרת הסל
| התוויה | תאריך הכללה | תחום קליני | Class Effect | מצב מחלה |
|---|---|---|---|---|
| מבוטח בגיר ומתבגר מגיל 13 ומעלה שהוא חולה סכיזופרניה, אשר עונה על אחד מהתנאים האלה: 1. פיתח תופעות לוואי לטיפול קודם ב-Aripiprazole; 2. הגיב חלקית לטיפול בתרופה אנטי פסיכוטית שניתנה לו כקו טיפול קודם, והוא מועמד לטיפול בתכשיר אנטי פסיכוטי מסוג D2 partial agonist; התחלת הטיפול בתרופה תהיה על פי הוראתו של רופא מומחה בפסיכיאטריה או בפסיכיאטריה של הילד והמתבגר, לפי העניין. לא יינתנו לחולה בו בזמן שתי תרופות או יותר ממשפחת התרופות האנטיפסיכוטיות האטיפיות. | 17/03/2024 | פסיכיאטריה | סכיזופרניה | |
| טיפול אוגמנטציה בדיכאון מסוג major depressive disorder (MDD), עבור חולים שפיתחו תופעות לוואי ל-Aripiprazole. התחלת הטיפול בתרופה תהיה על פי הוראתו של רופא מומחה בפסיכיאטריה. | 03/02/2022 | פסיכיאטריה | דיכאון, major depressive disorder, MDD | |
| מבוטח בגיר שהוא חולה סכיזופרניה, אשר עונה על אחד מהתנאים האלה: 1. פיתח תופעות לוואי לטיפול קודם ב-Aripiprazole; 2. הגיב חלקית לטיפול בתרופה אנטי פסיכוטית שניתנה לו כקו טיפול קודם, והוא מועמד לטיפול בתכשיר אנטי פסיכוטי מסוג D2 partial agonist | 01/03/2021 | פסיכיאטריה | סכיזופרניה, Schizophrenia |
שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
01/03/2021
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
עלון מידע לצרכן
02.05.21 - עלון לצרכן אנגלית 02.05.21 - עלון לצרכן עברית 02.05.21 - עלון לצרכן ערבית 11.02.24 - עלון לצרכן אנגלית 11.02.24 - עלון לצרכן עברית 20.02.24 - עלון לצרכן ערבית 09.09.24 - עלון לצרכן אנגלית 09.09.24 - עלון לצרכן עברית 27.11.24 - עלון לצרכן ערבית 05.07.20 - החמרה לעלון 19.07.20 - החמרה לעלון 14.02.24 - החמרה לעלון 09.09.24 - החמרה לעלוןלתרופה במאגר משרד הבריאות
ריקסלטי 4 מ"ג