Quest for the right Drug
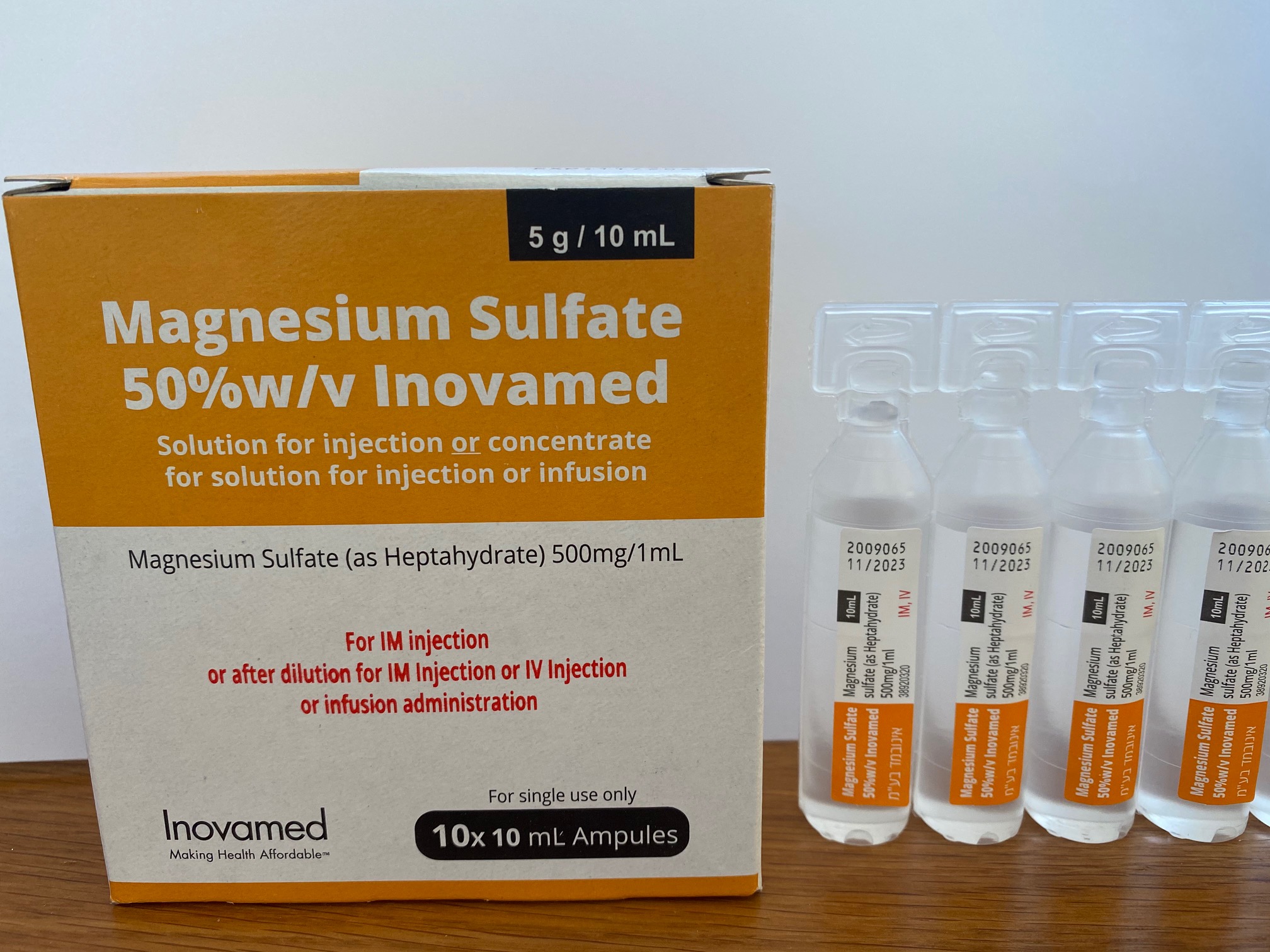
מגנזיום סולפאט 50%w/v אינובמד MAGNESIUM SULFATE 50 % W/V INOVAMED (MAGNESIUM SULFATE AS HEPTAHYDRATE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-ורידי, תוך-שרירי : I.V, I.M
צורת מינון:
אין פרטים : SOLUTION FOR INJECTION / CONCENTRATE FOR SOLUTION FOR INJ/INF
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Special Warning : אזהרת שימוש
4.4 Special warnings and precautions for use Magnesium salts should be administered with caution to patients with impaired renal function and appropriate dosage reduction should be made. Magnesium sulfate should not be used in hepatic coma if there is a risk of renal failure. Serum calcium levels should be routinely monitored in patients receiving magnesium sulfate. The serum-magnesium-level should be monitored during the treatment (normal 0.65 – 1.0 mmol). Monitoring of the absence of respiratory depression: the breath rate should not be under 16 breaths/min. The excretion of urine should not be under 25 mL/h, as it could lead to hypermagnesaemia (see sections 4.2 and 4.3). The presence of the patellar reflex should be checked. Administer with caution if flushing and sweating occurs. An antidote of injectable calcium gluconate solution should be immediately available. For the intravenous use in children the rate of administration should not exceed 0.02 mL/kg/min Magnesium sulfate 50% w/v Inovamed solution (corresponding to 0.04 mmol/kg/min = 0.001 g/kg/min Mg2+) diluted (see section 4.2). The 50% w/v solution MUST be diluted before use for IV administration; concentrations up to 20% w/v are usually employed. For IM use, a 25% w/v or 50% w/v solution is acceptable. For the intramuscular route, use good clinical practice for intramuscular injections. The 50% w/v solution should be used undiluted or diluted to 25% w/v. Avoid muscles which are emaciated or atrophied. Avoid the dorsogluteal muscle and sciatic nerve. If the total dose to be administered exceeds 5 mL, the injection volume should be divided between more than one deep muscular injection site. Use caution in older or thin patients who may only tolerate up to 2 mL in a single injection. Do not use an injection site that has evidence of infection or injury. If repeating an intramuscular dose, rotate injection sites to avoid injury or discomfort to the muscles.
Effects on Driving
4.7 Effects on ability to drive and use machines Magnesium Sulfate 50% w/v Inovamed solution is unlikely to affect the ability to drive or to operate machinery. However, some people may feel dizzy or drowsy when given Magnesium Sulfate 50% w/v Inovamed solution. The patient should be advised not to drive or operate machinery.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
עלון מידע לרופא
25.01.21 - עלון לרופאעלון מידע לצרכן
לתרופה במאגר משרד הבריאות
מגנזיום סולפאט 50%w/v אינובמד