Quest for the right Drug
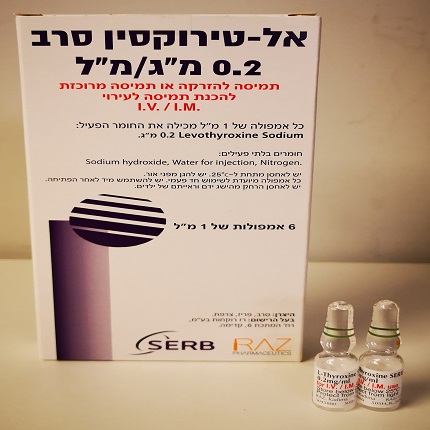
אל-טירוקסין סרב L-THYROXINE SERB (LEVOTHYROXINE SODIUM)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-שרירי, תוך-ורידי : I.M, I.V
צורת מינון:
אין פרטים : SOLUTION FOR INJECTION/ CONCENTRATE FOR SOLUTION FOR INFUSION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Posology : מינונים
4.2. Posology and method of administration Posology • Before treatment and in order to adjust the dose, it is recommended that testing of T3, T4 and TSH levels be performed. • The administered doses vary depending on the degree of hypothyroidism, the subject’s age and individual tolerance. • Daily administration of levothyroxine injection should be continued until the patient is able to tolerate an oral dose and is clinically stable. Adults Myxedema coma: An initial loading dose of 500 micrograms the first day is recommended, as a slow intravenous infusion in 250 ml of saline solution to achieve a concentration of the diluted solution of 2 micrograms/ml. Due to an increased risk of serious cardiovascular events or death, this loading dose must not exceed 500 µg. • Maintenance treatment should then be initiated at a daily dose of 100 µg on average. Replacement or supplemental therapy in congenital or acquired hypothyroidism of any etiology: • Gastrointestinal absorption of oral levothyroxine tablet is approximately 70%–80% in healthy fasting adults (see section 5.2). • Complete hormone replacement therapy in adults requires 100 to 150 μg as a single daily dose, on average. This dosage will be established gradually and with caution: start with 25 μg per day, then increase the daily dose by 25 μg at weekly intervals. • Once the dosage has been stable for a long enough period, repeat testing of thyroid hormones levels. Monitor T3 and T4 levels to check that there is no overdose and monitor normalisation of TSH levels in the event of peripheral hypothyroidism. Elderly patients More gradual dosing schedules may be proposed, particularly in elderly subjects with known cardiovascular risk factors (see section 4.4), for whom treatment should be initiated at lower doses, and follow more gradual increments. A maintenance dose lower than that required to normalise TSH levels may be considered. Patients with renal / hepatic insufficiency Experience in patients with renal and/or hepatic insufficiency is limited. Paediatric population Myxedema coma: Experience in children treated for myxedema coma is very limited. Replacement or supplemental therapy in congenital or acquired hypothyroidism of any etiology: The maintenance dose is generally 100 to 150 micrograms per m² of body surface area. • For neonates and infants with congenital hypothyroidism, in whom rapid replacement is important, the initial recommended dosage is 10 to 15 micrograms per kg bodyweight per day for the first 3 months. Thereafter, the dose should be individually adjusted based on clinical and laboratory findings (thyroid hormones and TSH). • For children with acquired hypothyroidism, the recommended initial dosage is 12.5 to 50 micrograms per day. The dose should be increased gradually every 2 to 4 weeks based on clinical and laboratory findings (thyroid hormones and TSH) until the full replacement dose is reached. In all cases, the dose should be adjusted on the basis of the needs of each individual. Method of administration Intravenous injection. Intramuscular injection possible. For the treatment of myxedema coma, a slow intravenous infusion in 250 ml of saline solution is recommended for the loading dose.

שימוש לפי פנקס קופ''ח כללית 1994
Thyroid deficiency states
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה שאושרה לשימוש כללי בקופ'ח
מידע נוסף
