Quest for the right Drug
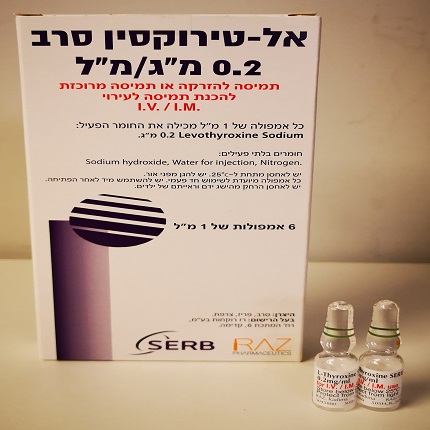
אל-טירוקסין סרב L-THYROXINE SERB (LEVOTHYROXINE SODIUM)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-שרירי, תוך-ורידי : I.M, I.V
צורת מינון:
אין פרטים : SOLUTION FOR INJECTION/ CONCENTRATE FOR SOLUTION FOR INFUSION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Special Warning : אזהרת שימוש
4.4. Special warnings and precautions for use Before starting a thyroid hormone therapy, the following diseases or conditions should be excluded or treated: - Coronary heart disease, - angina pectoris, - hypertension, - pituitary and/or adrenal insufficiency, - thyroid autonomy. It is essential that even mild, drug-induced hyperthyroidism be avoided in patients with coronary heart disease, heart failure, tachyarrhythmias, myocarditis of non-acute course, chronic hypothyroidism or in patients who have already suffered a myocardial infarction. In these patients, more frequent monitoring of thyroid hormone parameters is essential during thyroid hormone therapy (see section 4.2). Thyroid hormones should not be given for weight reduction. In euthyroid patients, treatment with levothyroxine does not cause weight reduction. Substantial doses may cause serious or even life-threatening undesirable effects, particularly in combination with certain substances for weight reduction, and especially with sympathomimetic amines. If a switch to another levothyroxine-containing product is required, there is a need to undertake a close monitoring including a clinical and biological monitoring during the transition period due to a potential risk of thyroid imbalance. In some patients, a dose adjustment could be necessary. Due to the difference of bioavailability of the oral dosage form versus injectable form, the dose should be carefully adapted when switching from one form to another (See section 4.2). Patients with cardiovascular disorders or with a history of cardiovascular disorders Levothyroxine by the intravenous/intramuscular route can be associated with cardiac toxicity (in particular arrhythmia, tachycardia, myocardial ischaemia and myocardial infarction or exacerbation of congestive heart failure and death) in patients with underlying cardiovascular disease (in particular coronary disorders, arrhythmias, hypertension, decompensated heart failure). Due to the increased prevalence of cardiovascular diseases in the elderly, caution is required when administering levothyroxine solution for injection/infusion in elderly patients or those with known cardiac risk factors. Cautious use may be required in these populations, including at doses at the lower end of the recommended dosage range (see section 4.2). Regular and careful monitoring of cardiac conditions is necessary at treatment initiation and throughout treatment. Patients with adrenal insufficiency In case of adrenocortical dysfunction, this should be treated before starting the therapy with levothyroxine by adequate replacement treatment to prevent acute adrenal insufficiency (See section 4.3). Low birth weight preterm neonates Haemodynamic parameters should be monitored when levothyroxine therapy is initiated in very low birth weight preterm neonates as circulatory collapse may occur due to the immature adrenal function. Diabetes The addition of levothyroxine to an anti-diabetic treatment or insulin therapy can lead to an increase in insulin or anti-diabetic drug requirements. Careful monitoring of metabolic control is recommended in diabetic patients (see section 4.5). Patients with a history of epilepsy Due to the risk of seizures in patients with a history of epilepsy, monitoring of these patients is recommended throughout treatment with levothyroxine. Hypersensitivity Hypersensitivity reactions (including angioedema), sometimes serious, have been reported with L-THYROXINE SERB. If signs and symptoms of allergic reactions occur, treatment with L-THYROXINE SERB must be discontinued and appropriate symptomatic treatment initiated (see Section 4.3 and 4.8). Pregnant women Clinical and laboratory monitoring must be reinforced at the most earliest stage possible in pregnant women, particularly during the first half of the pregnancy, in order to adjust the treatment if necessary (see section 4.6). Osteoporosis During levothyroxine therapy of postmenopausal women with increased risk of osteoporosis, dosage of levothyroxine sodium should be titrated to the lowest possible effective level and thyroid function should be monitored more frequently to avoid levels of levothyroxine above the physiological range (see section 4.8). Interferences with laboratory test: Biotin may interfere with thyroid immunoassays that are based on a biotin/streptavidin interaction, leading to either falsely decreased or falsely increased test results. The risk of interference increases with higher doses of biotin. When interpreting results of laboratory tests, possible biotin interference has to be taken into consideration, especially if a lack of coherence with the clinical presentation is observed. For patients taking biotin-containing products, laboratory personnel should be informed when a thyroid function test is requested. Alternative tests not susceptible to biotin interference should be used, if available (see section 4.5). Levothyroxine and other treatments: Monitoring is required in patients receiving concomitant administration of levothyroxine and medicinal products (such as amiodarone, tyrosine kinase inhibitors, salicylates and furosemide at high doses) which may affect the thyroid function. See also section 4.5. For diabetic patients and patients under anticoagulant therapy, see section 4.5. This medicine contains less than 1 mmol sodium (23 mg) per ampoule, that is to say essentially ‘sodium-free’.
Effects on Driving

שימוש לפי פנקס קופ''ח כללית 1994
Thyroid deficiency states
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה שאושרה לשימוש כללי בקופ'ח
מידע נוסף
