Quest for the right Drug
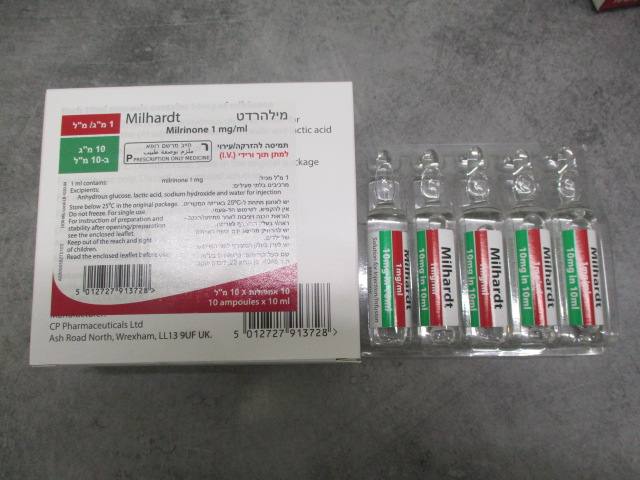
מילהרדט MILHARDT (MILRINONE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-ורידי : I.V
צורת מינון:
תמיסה להזרקהאינפוזיה : SOLUTION FOR INJECTION / INFUSION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmacological properties : תכונות פרמקולוגיות
Pharmacodynamic Properties
5.1 Pharmacodynamic properties Pharmacotherapeutic group: Cardiac therapy; Phosphodiesterase inhibitor, ATC code C01CE02 Milrinone is a positive inotrope and vasodilator, with little chronotropic activity. It also improves left ventricular diastolic relaxation. It differs in structure and mode of action from the digitalis glycosides, catecholamines or angiotensin-converting enzyme inhibitors. It is a selective inhibitor of peak III phosphodiesterase isoenzyme in cardiac and vascular muscle. It produces slight enhancement of A-V node conduction, but no other significant electro-physiological effects. In clinical studies Milhardt has been shown to produce prompt improvements in the haemodynamic indices of congestive heart failure, including cardiac output, pulmonary capillary wedge pressure and vascular resistance, without clinically significant effect on heart rate or myocardial oxygen consumption. Haemodynamic improvement during intravenous Milhardt therapy is accompanied by clinical symptomatic improvement in congestive cardiac failure, as measured by change in New York Heart Association classification. Paediatric population: Literature review identified clinical studies with patients treated for low cardiac output syndrome following cardiac surgery, septic shock or pulmonary hypertension. The usual dosages were a loading dose of 50 to 75 μg/kg administered over 30 to 60 minutes followed by an intravenous continuous infusion of 0.25 to 0.75 μg/kg/min for a period up to 35 hours. In these studies, milrinone demonstrated an increase of cardiac output, a decrease in cardiac filling pressure, a decrease in systemic and pulmonary vascular resistance, with minimal changes in heart rate and in myocardial oxygen consumption. Studies of a longer use of milrinone are not sufficient to recommend an administration of milrinone during a period of more than 35 hours. Some studies explored the paediatric use of milrinone in patients with non-hyperdynamic septic shock (Barton et al., 1996; Lindsay et al., 1998); the effect of milrinone on postbypass pulmonary hypertension after tetralogy of Fallot repair (Chu et al., 2000); the combined effect of nitric oxide and milrinone on pulmonary circulation after Fontan-type procedure (Cai et al., 2008). The results of these studies were inconclusive. Therefore, the use of milrinone in these indications cannot be recommended.
Pharmacokinetic Properties
5.2 Pharmacokinetic properties Following intravenous injections of 12.5 to 125 μg/kg to congestive heart failure patients, Milhardt had a volume of distribution of 0.38 l/kg/h, a mean terminal elimination half-life of 2.3 hours, and a clearance of 0.13 l/kg/h. Following intravenous infusions of 0.2 to 0.7 μg/kg/min to congestive heart failure patients, the drug had a volume of distribution of about 0.45 l/kg, a mean terminal elimination half-life of 2.4 hours, and a clearance of 0.14 l/kg/h. These pharmacokinetic parameters were not dose-dependent, and the area under the plasma concentration versus time curve following injection was significantly dose-dependent. The primary route of excretion of milrinone in man is via the urine. Elimination in normal subjects via the urine is rapid, with approximately 60% recovered within the first two hours following dosing, and approximately 90% recovered within the first eight hours following dosing. The mean renal clearance of milrinone is approximately 0.3 l/min, indicative of active secretion. Paediatric population: Milrinone is cleared more rapidly in children than in adults, but infants have significantly lower clearance than children, and preterm infants have even lower clearance. As a consequence of this more rapid clearance compared to adults, steady-state plasma concentrations of milrinone were lower in children than in adults. In paediatric population with normal renal function steady-state milrinone plasma concentrations after 6 to 12 hours continuous infusion of 0.5 to 0.75 μg/kg/min were about of 100 to 300 ng/ml. Following intravenous infusion of 0.5 to 0.75 μg/kg/min to neonates, infants and children after open-heart surgery, milrinone has a volume of distribution ranging from 0.35 to 0.9 litres/kg with no significant difference across age groups. Following intravenous infusion of 0.5 μg/kg/min to very preterm infants to prevent low systemic outflow after birth, milrinone has a volume of distribution of about 0.5 litres/kg. Several pharmacokinetic studies showed that, in paediatric population, clearance increases with increasing age. Infants have significantly lower clearance than children (3.4 to 3.8 ml/kg/min versus 5.9 to 6.7 ml/kg/min). In neonates milrinone clearance was about 1.64 ml/kg/min and preterm infants have even lower clearance (0.64 ml/kg/min). Milrinone has a mean terminal half-life of 2 to 4 hours in infants and children and a mean terminal elimination half-life of 10 hours in preterm infants. It was concluded that the optimal dose of milrinone in paediatric patients in order to obtain plasma levels above the threshold of pharmacodynamic efficacy appeared higher than in adults, but that optimal dose in preterms in order to obtain plasma levels above the threshold of pharmacodynamic efficacy appeared lower than in children. Patent ductus arteriosus: Milrinone is cleared by renal excretion and has a volume of distribution that is restricted to extracellular space which suggests that the fluid overload and hemodynamic changes associated with patent ductus arteriosus may have an effect on distribution and excretion of milrinone (see sections 4.2, 4.4, 4.8 and 5.3).

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
