Quest for the right Drug
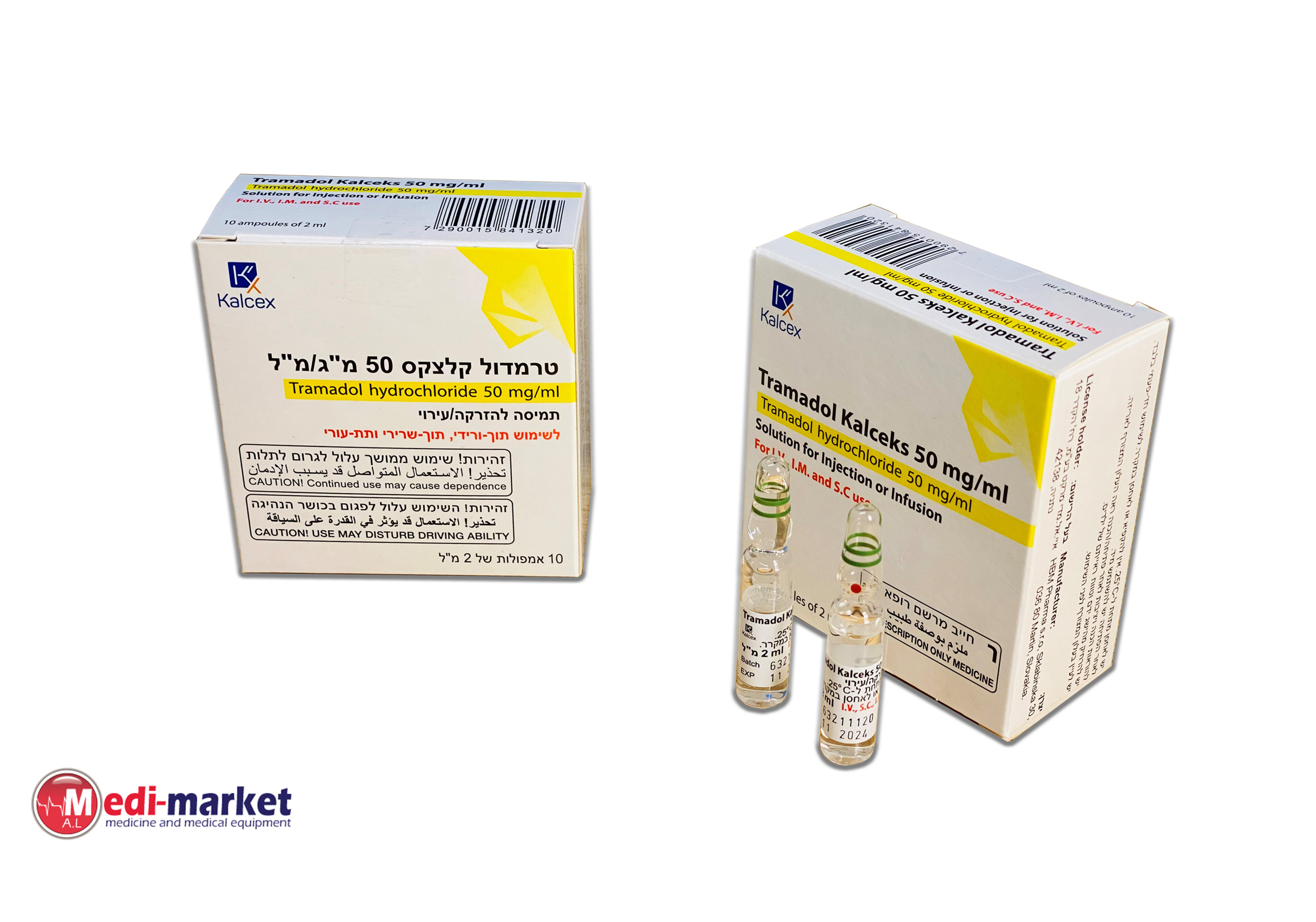
טרמדול - קלצקס 50 מ"ג/מ"ל TRAMADOL - KALCEKS 50 MG/ML (TRAMADOL HYDROCHLORIDE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תת-עורי, תוך-שרירי, תוך-ורידי : S.C, I.M, I.V
צורת מינון:
תמיסה להזרקהאינפוזיה : SOLUTION FOR INJECTION / INFUSION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Posology : מינונים
4.2 Posology and method of administration Prior to starting treatment with opioids, a discussion should be held with patients to put in place a strategy for ending treatment with tramadol in order to minimise the risk of addiction and drug withdrawal syndrome (see section 4.4). The injection is for parenteral administration either intramuscularly, by slow intravenous injection or, when diluted in solution, by infusion or patient controlled analgesia. As with all analgesic drugs the dosing of Tramadol- Kalceks should be adjusted depending on the severity of the pain and the individual clinical response of the patient. Adults: A single dose of 50 or 100 mg 4-6 hourly (1 or 2 ml of Tramadol-Kalceks) is usually required. Intravenous injections must be given slowly over 2-3 minutes. In severe (post-operative) pain, an initial bolus of 100 mg is administered 10- 20 minutes up to a total dose of 250 mg including the initial bolus. Subsequent doses should be 50 or 100 mg administered 4-6 hourly. If the administration of Tramadol-Kalceks has been forgotten, the pain may return. The dose should not be doubled. Administration should be continued as before. A total daily dose of 400 mg should not be exceeded except in special clinical circumstances. Tramadol-Kalceks is injected into the veins (usually into a blood vessel under the surface of the arm), muscles (usually the buttocks) or under the skin. Administration into the veins is slow with 1 ml Tramadol-Kalceks, solution for injection/infusion (equivalent to 50 mg tramadol hydrochloride) per minute. Alternatively, Tramadol-Kalceks may be diluted with a suitable infusion solution (e.g. 0.9% sodium chloride or 5% glucose solution) for i.v. infusion or for patient-controlled analgesia (PCA). Elderly patients: Dosing as for adults but it should be noted that in a study in elderly volunteers (aged over 75 years) the elimination half-life for orally administered tramadol was increased by 17%. Patients with renal insufficiency/renal dialysis: As the elimination of tramadol may be prolonged in patients with renal impairment, the usual initial adult doses should be employed, but prolongation of the dosage interval should be carefully considered according to the patient’s requirements. For creatinine clearance <30 ml/min the dosing should be increased to 12 hourly intervals. For creatinine clearance <10 ml/min (severe renal impairment) tramadol is not recommended. Tramadol is removed very slowly by haemodialysis or haemofiltration and therefore post dialysis dosing to maintain analgesia is usually unnecessary. Patients with hepatic insufficiency: It should be noted that as the elimination of tramadol may be prolonged in severe hepatic impairment, although the usual initial adult doses should be used, prolongation of the dosing should be at 12 hourly intervals. Children: Over 14 years: Dosage as for adults. Children aged 1 to 14 years: Receive 1-2 mg tramadol hydrochloride per kg body weight as a single dose. In this case Tramadol-Kalceks is diluted in water for injections. The following table shows which concentrations are achieved (1 ml Tramadol-Kalceks contains 50 mg tramadol hydrochloride): Diluting Tramadol-Kalceks Gives the In water for following injections concentrations: 2 ml + 2 ml 25.0 mg/ml 2 ml + 4 ml 16.7 mg/ml 2 ml + 6 ml 12.5 mg/ml 2 ml + 8 ml 10.0 mg/ml 2 ml + 10 ml 8.3 mg/ml 2 ml + 12 ml 7.1 mg/ml 2 ml + 14 ml 6.3 mg/ml 2 ml + 16 ml 5.6 mg/ml 2 ml + 18 ml 5.0 mg/ml Example: the doctor would like to give 1.5 mg tramadol hydrochloride per kg body weight to a child weighing 45 kg. This requires 67.5 mg tramadol hydrochloride. Therefore 2 ml Tramadol-Kalceks are diluted in 4 ml water for injection. This results in a concentration of 16.7 mg tramadol hydrochloride per ml. 4 ml (approx. 67 mg tramadol hydrochloride) of the diluted solution are then administered. If you interrupt or stop treatment with Tramadol-Kalceks too soon, pain is likely to return. In general stopping treatment with Tramadol-Kalceks will have no after-effects. However, in some patients given Tramadol-Kalceks for a very long time and then stopped suddenly, after-effects may occur. The patients might feel restless, anxious, nervous or dithery. They might be overactive, sleep badly, or have stomach or bowel trouble. A very small number of people might have panic attacks, hallucinations, abnormal sensations such as tingling and numbness, or ringing in the ears (tinnitus). Method of administration The solution for injection/infusion is to be injected slowly or diluted in infusion solution and infused. For instructions on dilution of the medicinal product before administration, see section 6.6. Duration of administration Tramadol-Kalceks should under no circumstances be administered for longer than absolutely necessary. If long-term pain treatment with tramadol is necessary in view of the nature and severity of the illness, then careful and regular monitoring should be carried out (if necessary with breaks in treatment) to establish whether and to what extent further treatment is necessary.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
