Quest for the right Drug
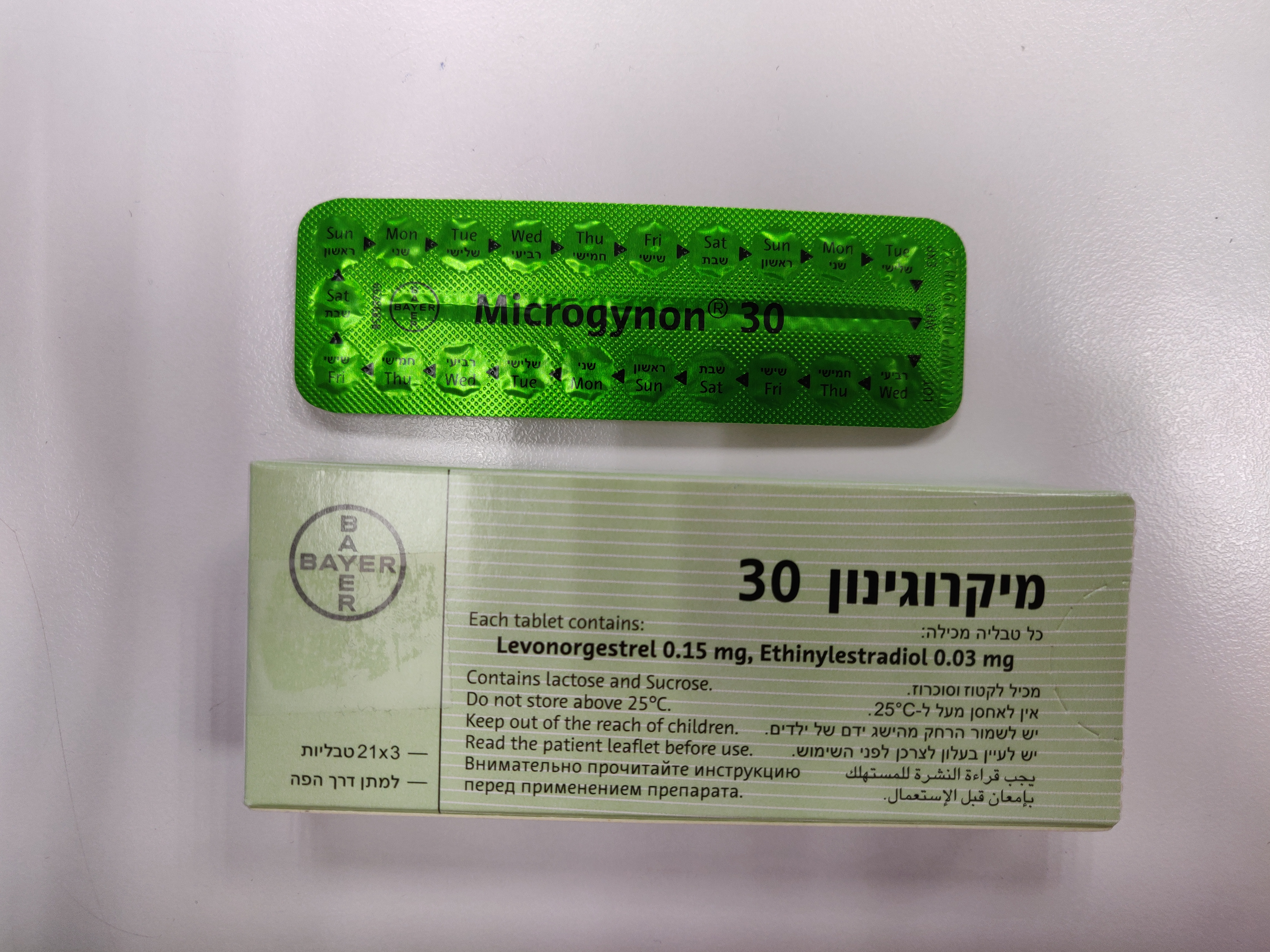
מיקרוגינון 30 MICROGYNON ® 30 (ETHINYLESTRADIOL, LEVONORGESTREL)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : PER OS
צורת מינון:
טבליות מצופות : COATED TABLETS
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Special Warning : אזהרת שימוש
4.4. Special warnings and precautions for use Warnings • If any of the conditions or risk factors mentioned below is present, the suitability of Microgynon 30 should be discussed with the woman. • In the event of aggravation, or first appearance of any of these conditions or risk factors, the woman should be advised to contact her doctor to determine whether the use of Microgynon 30 should be discontinued. Risk of venous thromboembolism (VTE) The use of any combined hormonal contraceptive (CHC) increases the risk of venous thromboembolism (VTE) compared with no use. Products that contain levonorgestrel, such as Microgynon 30, norgestimate or norethisterone are associated with the lowest risk of VTE. The decision to use Microgynon 30 should be taken after a discussion with the woman to ensure she understands the risk of VTE with Microgynon 30, how her current risk factors influence this risk, and that her VTE risk is highest in the first ever year of use. There is also some evidence that the risk is increased when a CHC is re-started after a break in use of 4 weeks or more. In women who do not use a CHC and are not pregnant, about 2 out of 10,000 will develop a VTE over the period of one year. However, in any individual woman the risk may be far higher, depending on her underlying risk factors (see below). It is estimated that out of 10,000 women who use a CHC that contains levonorgestrel, about 61 will develop a VTE in a year. This number of VTEs per year is fewer than the number expected in women during pregnancy or in the postpartum period. VTE may be fatal in 1-2 % of cases. ________ 1 Mid-point of range of 5-7 per 10,000 WY, based on a relative risk for CHCs containing levonorgestrel versus non-use of approximately 2.3 to 3.6. Number of VTE events per 10,000 women in one year Extremely rarely, thrombosis has been reported to occur in CHC users in other blood vessels, e.g. hepatic, mesenteric, renal, cerebral or retinal veins and arteries. Risk factors for VTE The risk for venous thromboembolic complications in CHC users may increase substantially in a woman with additional risk factors, particularly if there are multiple risk factors (see table). Microgynon 30 is contraindicated if a woman has multiple risk factors that put her at high risk of venous thrombosis (see section 4.3). If a woman has more than one risk factor, it is possible that the increase in risk is greater than the sum of the individual factors – in this case her total risk of VTE should be considered. If the balance of benefits and risks is considered to be negative a CHC should not be prescribed (see section 4.3). Table: Risk factors for VTE Risk factor Comment Obesity (body mass index over 30 kg/m²) Risk increases substantially as BMI rises. Particularly important to consider if other risk factors also present. Prolonged immobilisation, major surgery, any In these situations it is advisable to discontinue surgery to the legs or pelvis, neurosurgery, or use of the pill (in the case of elective surgery at major trauma least four weeks in advance) and not resume until two weeks after complete remobilisation. Another method of contraception should be Note: temporary immobilisation including air used to avoid unintentional pregnancy. travel >4 hours can also be a risk factor for VTE, particularly in women with other risk Antithrombotic treatment should be considered factors. if Microgynon 30 has not been discontinued in advance. Positive family history (venous If a hereditary predisposition is suspected, the thromboembolism ever in a sibling or parent woman should be referred to a specialist for especially at a relatively early age e.g. before advice before deciding about any CHC use. 50). Other medical conditions associated with VTE Cancer, systemic lupus erythematosus, haemolytic uraemic syndrome, chronic inflammatory bowel disease (Crohn’s disease or ulcerative colitis) and sickle cell disease. Increasing age Particularly above 35 years. There is no consensus about the possible role of varicose veins and superficial thrombophlebitis in the onset or progression of venous thrombosis. The increased risk of thromboembolism in pregnancy, and particularly the 6 week period of the puerperium, must be considered (for information on “Pregnancy and lactation” see Section 4.6). Symptoms of VTE (deep vein thrombosis and pulmonary embolism) In the event of symptoms women should be advised to seek urgent medical attention and to inform the healthcare professional that she is taking a CHC. Symptoms of deep vein thrombosis (DVT) can include: − unilateral swelling of the leg and/or foot or along a vein in the leg; − pain or tenderness in the leg which may be felt only when standing or walking, − increased warmth in the affected leg; red or discoloured skin on the leg. Symptoms of pulmonary embolism (PE) can include: − sudden onset of unexplained shortness of breath or rapid breathing; − sudden coughing which may be associated with haemoptysis; − sharp chest pain; − severe light headedness or dizziness; − rapid or irregular heartbeat. Some of these symptoms (e.g. “shortness of breath”, “coughing”) are non-specific and might be misinterpreted as more common or less severe events (e.g. respiratory tract infections). Other signs of vascular occlusion can include: sudden pain, swelling and slight blue discoloration of an extremity. If the occlusion occurs in the eye symptoms can range from painless blurring of vision which can progress to loss of vision. Sometimes loss of vision can occur almost immediately. Risk of arterial thromboembolism (ATE) Epidemiological studies have associated the use of CHCs with an increased risk for arterial thromboembolism (myocardial infarction) or for cerebrovascular accident (e.g. transient ischaemic attack, stroke). Arterial thromboembolic events may be fatal. Risk factors for ATE The risk of arterial thromboembolic complications or of a cerebrovascular accident in CHC users increases in women with risk factors (see table). Microgynon 30 is contraindicated if a woman has one serious or multiple risk factors for ATE that puts her at high risk of arterial thrombosis (see section 4.3). If a woman has more than one risk factor, it is possible that the increase in risk is greater than the sum of the individual factors - in this case her total risk should be considered. If the balance of benefits and risks is considered to be negative a CHC should not be prescribed (see section 4.3). Table: Risk factors for ATE Risk factor Comment Increasing age Particularly above 35 years Smoking Women should be advised not to smoke if they wish to use a CHC. Women over 35 who continue to smoke should be strongly advised to use a different method of contraception. Hypertension Obesity (body mass index over 30 kg/m2) Risk increases substantially as BMI increases. Particularly important in women with additional risk factors Positive family history (arterial If a hereditary predisposition is suspected, the thromboembolism ever in a sibling or parent woman should be referred to a specialist for especially at relatively early age e.g. below 50). advice before deciding about any CHC use Migraine An increase in frequency or severity of migraine during CHC use (which may be prodromal of a cerebrovascular event) may be a reason for immediate discontinuation Other medical conditions associated with Diabetes mellitus, hyperhomocysteinaemia, adverse vascular events valvular heart disease and atrial fibrillation, dyslipoproteinaemia and systemic lupus erythematosus. Symptoms of ATE In the event of symptoms women should be advised to seek urgent medical attention and to inform the healthcare professional that she is taking a CHC. Symptoms of a cerebrovascular accident can include: − sudden numbness or weakness of the face, arm or leg, especially on one side of the body; − sudden trouble walking, dizziness, loss of balance or coordination; − sudden confusion, trouble speaking or understanding; − sudden trouble seeing in one or both eyes; − sudden, severe or prolonged headache with no known cause; − loss of consciousness or fainting with or without seizure. Temporary symptoms suggest the event is a transient ischaemic attack (TIA). Symptoms of myocardial infarction (MI) can include: − pain, discomfort, pressure, heaviness, sensation of squeezing or fullness in the chest, arm, or below the breastbone; − discomfort radiating to the back, jaw, throat, arm, stomach; − feeling of being full, having indigestion or choking; − sweating, nausea, vomiting or dizziness; − extreme weakness, anxiety, or shortness of breath; − rapid or irregular heartbeats. Medical Examination/Consultation Prior to the initiation or reinstitution of Microgynon 30 a complete medical history (including family history) should be taken and pregnancy must be ruled out. Blood pressure should be measured and a physical examination should be performed, guided by the contra-indications (see section 4.3) and warnings (see section 4.4). It is important to draw a woman’s attention to the information on venous and arterial thrombosis, including the risk of Microgynon 30 compared with other CHCs, the symptoms of VTE and ATE, the known risk factors and what to do in the event of a suspected thrombosis. The woman should also be instructed to carefully read the user leaflet and to adhere to the advice given. The frequency and nature of examinations should be based on established practice guidelines and be adapted to the individual woman. Women should be advised that hormonal contraceptives do not protect against HIV infections (AIDS) and other sexually transmitted diseases. Undiagnosed vaginal bleeding that is suspicious for underlying conditions should be investigated. Conditions which require strict medical supervision The decision to prescribe the COC must be made using clinical judgement and in consultation with the woman. Exacerbation or first appearance of any of these conditions or risk factors may indicate that use of the oral contraceptive should be discontinued. The woman should contact her physician, who should then decide on whether COC use should be discontinued: • Diabetes mellitus with mild vascular disease or mild nephropathy, retinopathy or neuropathy • Hypertension that is adequately controlled, i.e. systolic >140 to159 mm Hg or diastolic > 90 to 94 mm Hg (see also Section 4.4 ‘Reasons for stopping oral contraception immediately’) • porphyria • obesity • migraine • cardiovascular diseases Reasons for stopping oral contraception immediately: When stopping oral contraception non-hormonal contraception should be used to ensure contraceptive protection is maintained. 1. Occurrence for the first time, or exacerbation, of migrainous headaches or unusually frequent or unusually severe headaches 2. Sudden disturbances of vision, of hearing or other perceptual disorders 3. First signs of thrombosis or blood clots (e.g. unusual pains in or swelling of the leg(s), stabbing pains on breathing or coughing for no apparent reason). Feeling of pain and tightness in the chest 4. At least four weeks before an elective major operation (e.g. abdominal, orthopaedic), any surgery to the legs, medical treatment for varicose veins or prolonged immobilisation, e.g. after accidents or surgery. Do not restart until 2 weeks after full ambulation. In case of emergency surgery, thrombotic prophylaxis is usually indicated e.g. subcutaneous heparin 5. Onset of jaundice, hepatitis, itching of the whole body 6. Significant rise in blood pressure 7. Severe upper abdominal pain or liver enlargement 8. Clear exacerbation of conditions known to be capable of deteriorating during oral contraception or pregnancy (see section 4.4 ‘Conditions which deteriorate in pregnancy or during previous COC use’ under 'Other conditions') Tumours Numerous epidemiological studies have been reported on the risks of ovarian, endometrial, cervical and breast cancer in women using combined oral contraceptives. The evidence is clear that high dose combined oral contraceptives offer substantial protection against both ovarian and endometrial cancer. However, it is not clear whether low dose COCs confer protective effects to the same level. • Breast cancer A meta-analysis from 54 epidemiological studies reported that there is a slightly increased relative risk (RR = 1.24) of having breast cancer diagnosed in women who are currently using combined oral contraceptives (COCs). The observed pattern of increased risk may be due to an earlier diagnosis of breast cancer in COC users, the biological effects of COCs or a combination of both. The additional breast cancers diagnosed in current users of COCs or in women who have used COCs in the last ten years are more likely to be localised to the breast than those in women who never used COCs. Breast cancer is rare among women under 40 years of age whether or not they take COCs. Whilst this background risk increases with age, the excess number of breast cancer diagnoses in current and recent COC users is small in relation to the overall risk of breast cancer (see bar chart). The most important risk factor for breast cancer in COC users is the age women discontinue the COC; the older the age at stopping, the more breast cancers are diagnosed. Duration of use is less important and the excess risk gradually disappears during the course of the 10 years after stopping COC use such that by 10 years there appears to be no excess. The possible increase in risk of breast cancer should be discussed with the user and weighed against the benefits of COCs taking into account the evidence that they offer substantial protection against the risk of developing certain other cancers (e.g. ovarian and endometrial cancer). • Cervical Cancer The most important risk factor for cervical cancer is persistent HPV infection. Some epidemiological studies have indicated that long-term use of COCs may further contribute to this increased risk but there continues to be controversy about the extent to which this finding is attributable to confounding effects, e.g., cervical screening and sexual behaviour including use of barrier contraceptives. • Liver Cancer In rare cases benign and, in even rarer cases, malignant liver tumours leading in isolated cases to life-threatening intra-abdominal haemorrhage have been observed after the use of hormonal substances such as those contained in Microgynon 30. If severe upper abdominal complaints, liver enlargement or signs of intra-abdominal haemorrhage occur, the possibility of a liver tumour should be included in the differential diagnosis. Other conditions The possibility cannot be ruled out that certain chronic diseases may occasionally deteriorate during the use of combined oral contraceptives. • Known hyperlipidaemias Women with hypertriglyceridemia, or a family history thereof, may be at an increased risk of pancreatitis when using COCs. Women with hyperlipidaemias are at an increased risk of arterial disease (see section 4.4 ‘Risk of arterial thromboembolism (ATE)’). However routine screening of women on COCs is not appropriate. • Blood pressure Hypertension is a risk factor for stroke and myocardial infarction (see section 4.4 ‘Risk of arterial thromboembolism (ATE)’).Although small increases in blood pressure have been reported in many women taking COCs, clinically relevant increases are rare. However, if sustained hypertension develops during the use of a COC, antihypertensive treatment should normally be instigated at a level of 160/100 mm Hg in uncomplicated patients or at 140/90 mm Hg in those with target organ damage, established cardiovascular disease, diabetes or with increased cardiovascular risk factors. Decisions about the continued use of the COC should be made at lower BP levels, and alternative contraception may be advised. • Conditions which deteriorate in pregnancy or during previous COC use The following conditions have been reported to occur or deteriorate with both pregnancy and COC use. Consideration should be given to stopping Microgynon 30 if any of the following occur during use: • jaundice and/or pruritus related to cholestasis • COCs may increase the risk of gallstone formation and may worsen existing disease. • systemic lupus erythematosus • herpes gestationis • otosclerosis-related hearing loss • sickle cell anaemia • renal dysfunction • hereditary angioedema • any other condition an individual woman has experienced worsening of during pregnancy or previous use of COCs. • Angioedema Exogenous oestrogens may induce or exacerbate symptoms of hereditary and acquired angioedema. • Disturbances of liver function Acute or chronic disturbances of liver function may necessitate the discontinuation of COC use until markers of liver function return to normal. Recurrence of cholestatic jaundice and/or cholestasis-related pruritus which occurred during pregnancy or previous use of sex steroids necessitates the discontinuation of COCs. • Diabetes (without vascular involvement) Insulin-dependent diabetics without vascular disease can use COCs. However it should be remembered that all diabetics are at an increased risk of arterial disease and this should be considered when prescribing COCs. Diabetics with existing vascular disease are contraindicated from using COCs (see section 4.3 Contraindications). Although COCs may have an effect on peripheral insulin resistance and glucose tolerance, there is no evidence for a need to alter the therapeutic regimen in diabetics using low-dose COCs (containing < 0.05 mg ethinylestradiol). However, diabetic women should be carefully observed while taking COCs. • Psychiatric disorders Depressed mood and depression are well-known undesirable effects of hormonal contraceptive use (see section 4.8). Depression can be serious and is a well-known risk factor for suicidal behaviour and suicide. Women should be advised to contact their physician in case of mood changes and depressive symptoms, including shortly after initiating the treatment. • Chloasma Chloasma may occasionally occur, especially in women with a history of chloasma gravidarum. Women with a tendency to chloasma should avoid exposure to the sun or ultraviolet radiation whilst taking COCs. • Menstrual Changes Reduction of menstrual flow: This is not abnormal and it is to be expected in some patients. Indeed, it may be beneficial where heavy periods were previously experienced. Missed menstruation: Occasionally, withdrawal bleeding may not occur at all. If the tablets have been taken correctly, pregnancy is very unlikely. If withdrawal bleeding fails to occur at the end of a second pack, the possibility of pregnancy must be ruled out before resuming with the next pack. Intermenstrual bleeding: Irregular bleeding (spotting or breakthrough bleeding) may occur especially during the first months of use. Therefore, the evaluation of any irregular bleeding is only meaningful after an adaptation interval of about three cycles. If bleeding irregularities persist or occur after previously regular cycles, then non-hormonal causes should be considered and adequate diagnostic measures are indicated to exclude malignancy or pregnancy. This may include curettage. Some women may experience amenorrhoea or oligomenorrhoea after discontinuation of oral contraceptives, especially when these conditions existed prior to use. Women should be informed of this possibility. • Lactose and Sucrose Intolerance Each tablet of this medicinal product contains 32.82 mg lactose and 19.371 mg sucrose per tablet. Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency, fructose intolerance or glucose-galactose malabsorption or sucrase-isomaltase should not take this medicine. • Reduced efficacy The efficacy of COCs may be reduced, in the event of missed tablets , vomiting or diarhhoea, or concomitant medication.
Effects on Driving

שימוש לפי פנקס קופ''ח כללית 1994
Contraception
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה שאושרה לשימוש כללי בקופ'ח
מידע נוסף
עלון מידע לצרכן
18.01.16 - עלון לצרכן 09.03.22 - עלון לצרכן אנגלית 09.03.22 - עלון לצרכן עברית 09.03.22 - עלון לצרכן ערבית 11.10.22 - עלון לצרכן עברית 11.06.23 - עלון לצרכן 06.03.23 - עלון לצרכן אנגלית 06.03.23 - עלון לצרכן ערבית 30.08.23 - עלון לצרכן אנגלית 30.08.23 - עלון לצרכן עברית 30.08.23 - עלון לצרכן ערבית 14.11.19 - החמרה לעלון 13.09.20 - החמרה לעלון 24.06.21 - החמרה לעלון 11.10.22 - החמרה לעלון 11.06.23 - החמרה לעלוןלתרופה במאגר משרד הבריאות
מיקרוגינון 30