Quest for the right Drug
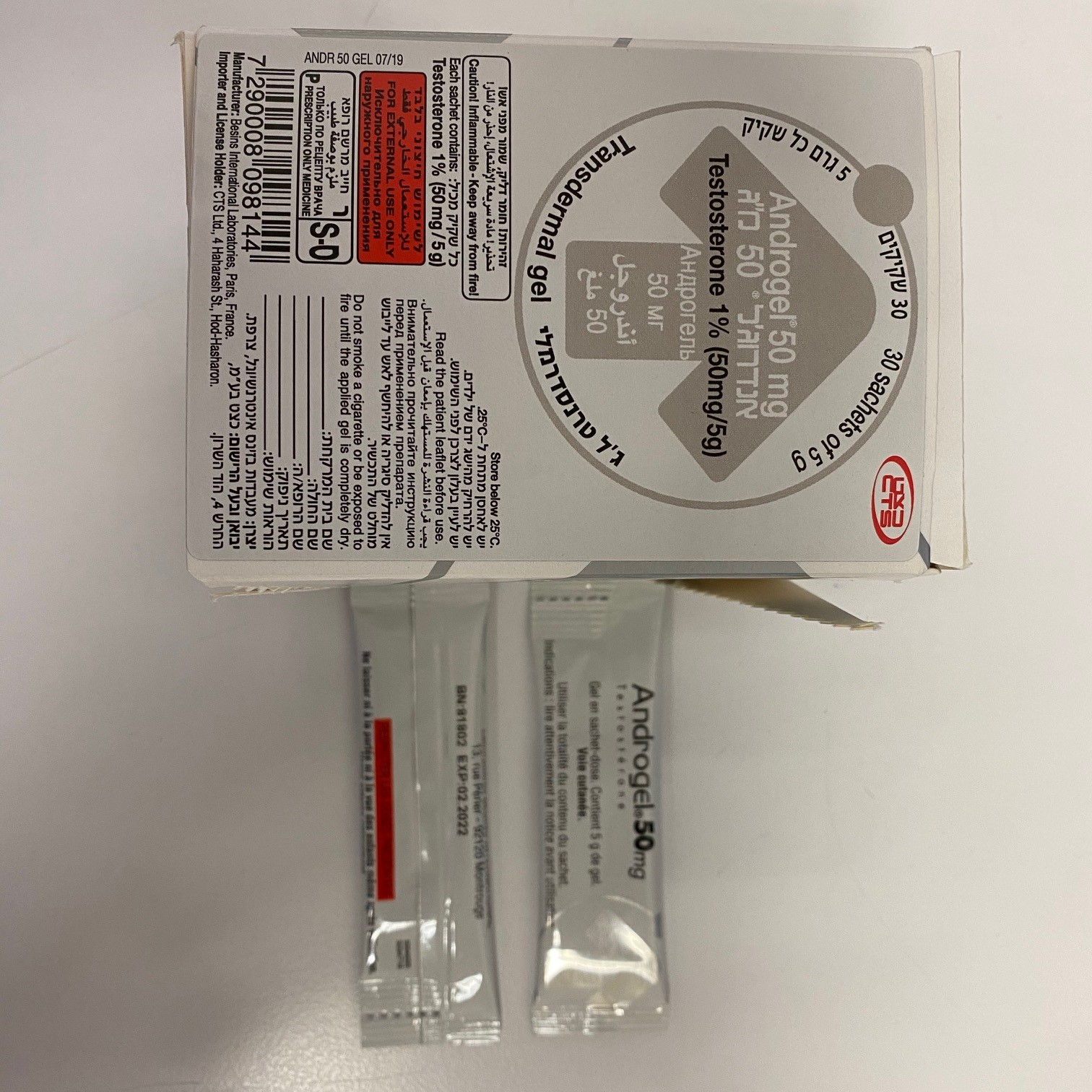
אנדרוג'ל 50 מ"ג ANDROGEL 50 MG (TESTOSTERONE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
בין-עורי : TRANSDERMAL
צורת מינון:
ג'ל : GEL
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmacological properties : תכונות פרמקולוגיות
Pharmacodynamic Properties
5.1 Pharmacodynamic properties Pharmacotherapeutic group: ANDROGENS, ATC Code: G03B A03 Endogenous androgens, principally testosterone, secreted by the testes and its major metabolite DHT, are responsible for the development of the external and internal genital organs and for maintaining the secondary sexual characteristics (stimulating hair growth, deepening of the voice, development of the libido); for a general effect on protein anabolism; for development of skeletal muscle and body fat distribution; for a reduction in urinary nitrogen, sodium, potassium, chloride, phosphate and water excretion. Testosterone does not produce testicular development: it reduces the pituitary secretion of gonadotropins. The effects of testosterone in some target organs arise after peripheral conversion of testosterone to estradiol, which than binds to oestrogen receptors in the target cell nucleus e.g. the pituitary, fat, brain, bone and testicular Leydig cells.
Pharmacokinetic Properties
5.2 Pharmacokinetic properties The percutaneous absorption of testosterone ranges from approximately 9% to 14% of the applied dose. Following percutaneous absorption, testosterone diffuses into the systemic circulation at relatively constant concentrations during the 24-hour cycle. Serum testosterone concentrations increase from the first hour after an application, reaching steady state from day two. Daily changes in testosterone concentrations are then of similar amplitude to those observed during the circadian rhythm of endogenous testosterone. The percutaneous route therefore avoids the blood distribution peaks produced by injections. It does not produce supra-physiological hepatic concentrations of the steroid in contrast to oral androgen therapy. Administration of 5 g of this medicine produces an average testosterone concentration increase of approximately 2.5 ng/ml (8.7 nmol/l) in plasma. When treatment is stopped, testosterone concentrations start decreasing approximately 24 hours after the last dose. Concentrations return to baseline approximately 72 to 96 hours after the final dose. The major active metabolites of testosterone are dihydrotestosterone and estradiol. Testosterone is excreted, mostly in urine, and in faeces as conjugated testosterone metabolites.

שימוש לפי פנקס קופ''ח כללית 1994
Androgen deficiency states in men, breast cancer in women, aplastic anemia
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה שאושרה לשימוש כללי בקופ'ח
מידע נוסף
