Quest for the right Drug
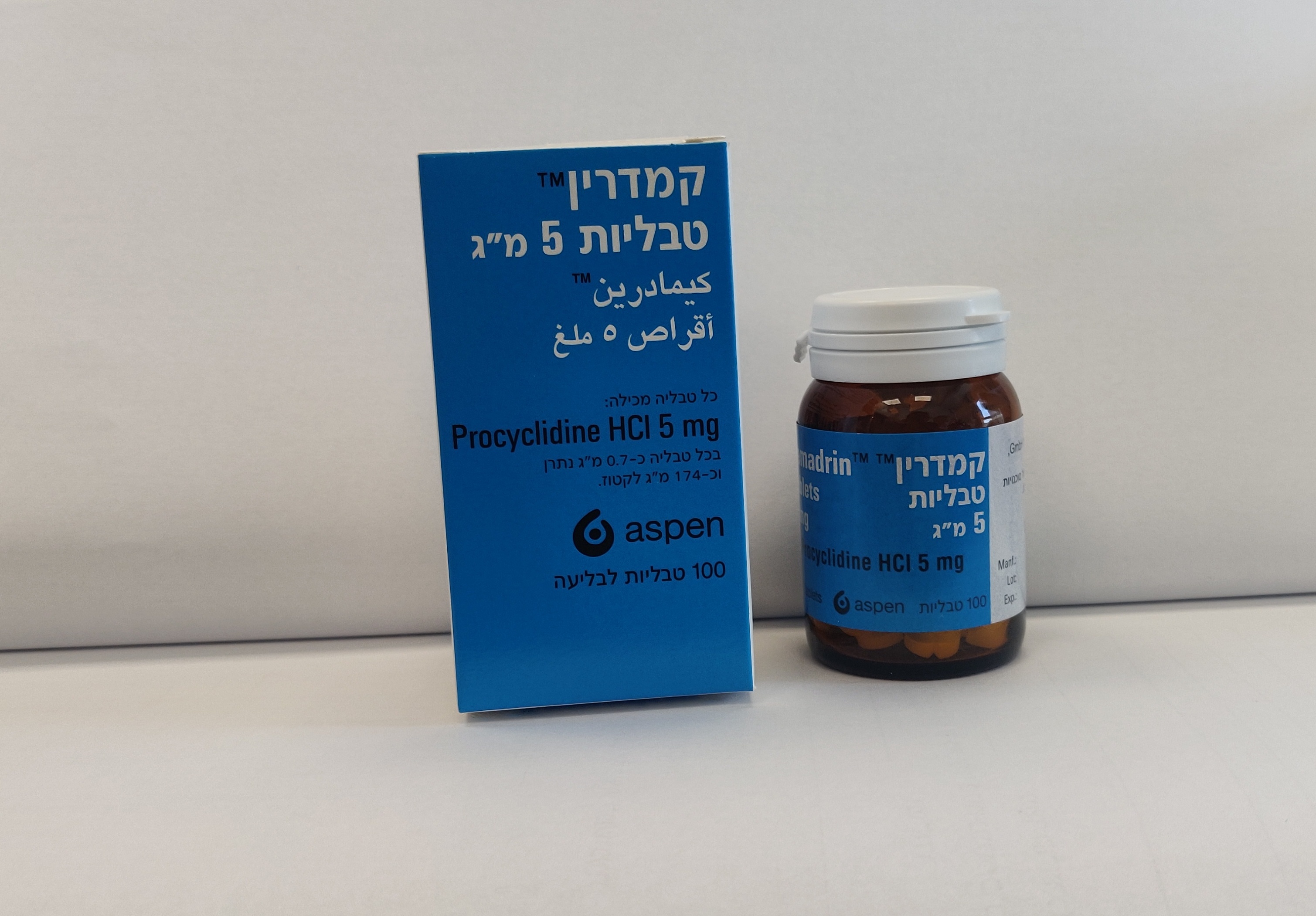
קמדרין טבליות 5 מ"ג KEMADRIN TABLETS 5 MG (PROCYCLIDINE HYDROCHLORIDE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : PER OS
צורת מינון:
טבליה : TABLETS
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Special Warning : אזהרת שימוש
4.4 Special warnings and precautions for use As with all anticholinergics the benefit/risk ratio should be assessed when prescribing Kemadrin in patients with existing angle-closure (narrow angle) glaucoma or those considered to be predisposed to glaucoma. Cautious prescribing is also indicated in patients predisposed to obstructive disease of the gastro-intestinal tract and those with urinary symptoms associated with prostatic hypertrophy. In a proportion of patients undergoing neuroleptic treatment, tardive dyskinesias will occur. While anti-cholinergic agents do not cause this syndrome, when given in combination with neuroleptics they may exacerbate the symptoms of tardive dyskinesia or reduce the threshold at which these symptoms appear in predisposed patients. In such individuals subsequent adjustment of neuroleptic therapy or reduction in anticholinergic treatment should be considered. Elderly patients, especially those on high doses of anticholinergics may be more susceptible to the adverse events associated with such therapy (see section 4.8). Specifically, the elderly patient may be particularly vulnerable to Central Nervous System disturbances such as confusion, impairment of cognitive function and memory, disorientation and hallucinations. These effects are usually reversible on reduction or discontinuation of anticholinergic therapy. There is no specific information available concerning the use of procyclidine hydrochloride in patients with impaired renal or hepatic function. However, since procyclidine is metabolised in the liver and excreted via the urine care should be exercised when administering procyclidine to patients with impairment of renal or hepatic function. Kemadrin should not be withdrawn abruptly as rebound parkinsonian symptoms may occur. Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take this medicine. Abuse Kemadrin, along with other anticholinergic drugs, has the potential to be abused. Although the cases of abuse are rare, physicians should exercise caution in prescribing Kemadrin to patients with symptoms that may not be genuine.
Effects on Driving
4.7 Effects on ability to drive and use machines Adverse events of a neurological character such as blurred vision, dizziness, confusion and disorientation have been reported with procyclidine. Therefore, if affected, patients should be advised not to drive or operate machinery.

שימוש לפי פנקס קופ''ח כללית 1994
Parkinsonism, drug induced extrapyramidal symptoms, (הערה: more beneficial effects in rigidity than in tremor)
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה שאושרה לשימוש כללי בקופ'ח
מידע נוסף
