Quest for the right Drug
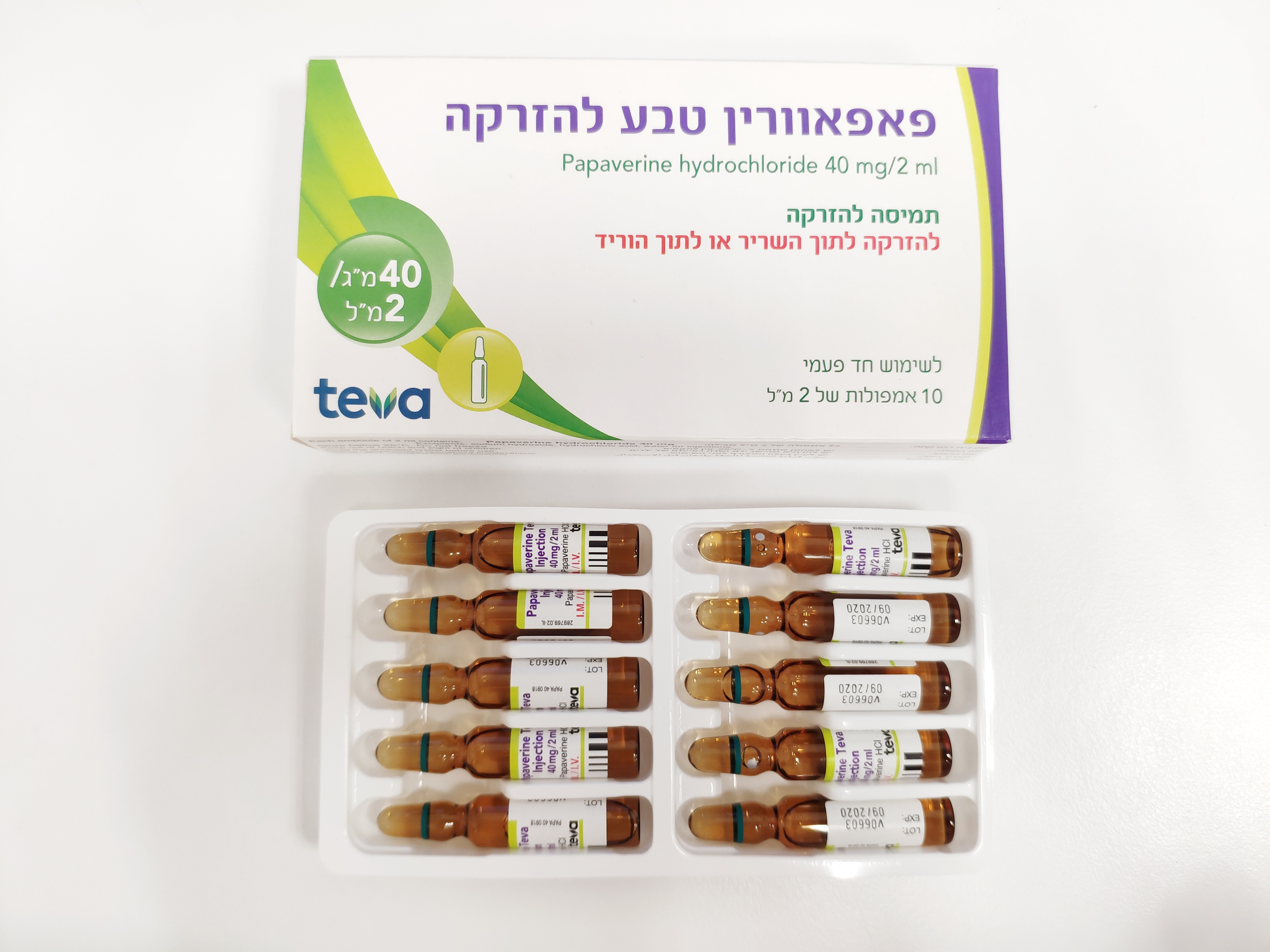
פאפאוורין טבע להזרקה PAPAVERINE TEVA INJECTION (PAPAVERINE HYDROCHLORIDE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-שרירי, תוך-ורידי : I.M, I.V
צורת מינון:
תמיסה להזרקה : SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8 Undesirable effects The adverse effects of papaverine described in the literature are listed below and classified by system organ class and frequency. The frequencies are defined as follows: very common (≥1/10); common (≥1/100, <1/10); uncommon (≥1/1,000, <1/100); rare (≥1/10,000, <1/1,000); very rare (<1/10,000); not known (cannot be estimated from the available data). Table: Known side effects System organ class Undesirable effects Frequency Nervous system disorders increased depth of breathing, Rare (Autonomic Nervous System) depression, dizziness, lightheadedness, headache, drowsiness, sedation, lassitude, alertness disorder, malaise, weakness and lethargy. Cardiac disorders increased heart rate, arrhythmias Rare (too rapid injection or injection of too high doses), atrioventricular block, tachycardia Vascular disorders hypotension or increased blood Rare pressure Gastrointestinal disorders* constipation, nausea, diarrhea, Rare abdominal distress and anorexia, vomiting Skin and subcutaneous pruritus, rash, Rare disorders Hepatobiliary disorders Hepatotoxicity: Hepatitis and Not known increased hepatic enzymes (alkaline phosphatase, SGOT) suggesting hepatotoxicity. General disorders and Hypersensitivity reactions Very rare administration site conditions General discomfort, redness in Rare the face, sweating, dry mouth and throat Thrombosis at the injection site. Not known * subjects with bowel movement disorders are more easily exposed to digestive disorders. Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form https://sideeffects.health.gov.il

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
