Quest for the right Drug
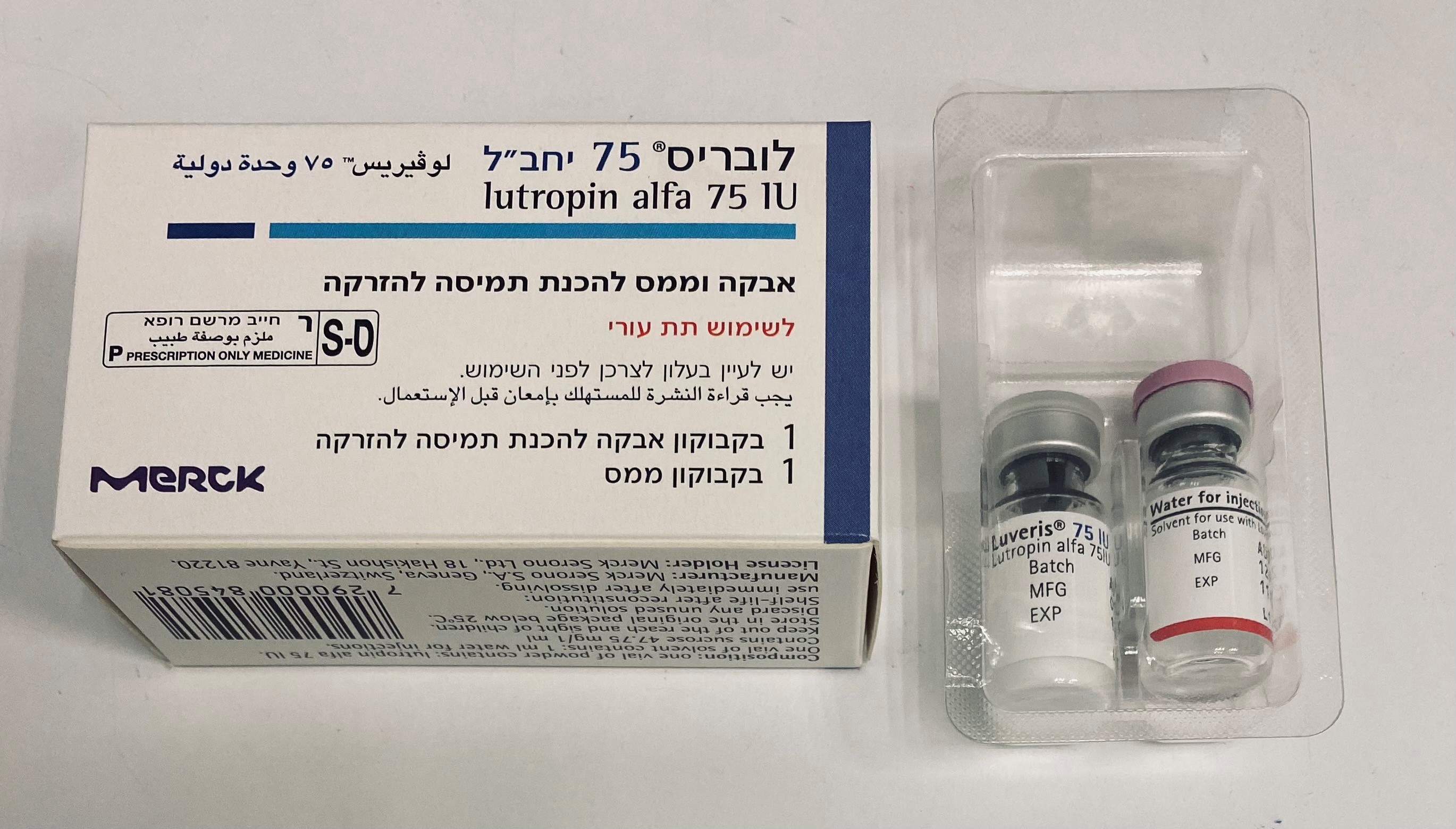
לובריס 75 LUVERIS 75 IU (LUTEINIZING HORMONE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תת-עורי : S.C
צורת מינון:
אבקה להכנת תמיסה לזריקה : POWDER FOR SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8 Undesirable effects Summary of the safety profile Luveris is used for the stimulation of follicular development in association with follitropin alfa. In this context, it is difficult to attribute adverse reactions to any one of the substances used. In a clinical trial, mild and moderate injection site reactions (bruising, pain, redness, itching or swelling) were reported in 7.4% and 0.9% of the injections, respectively. No severe injection site reactions were reported. Ovarian hyperstimulation syndrome (OHSS) was observed in less than 6% of patients treated with Luveris. No severe OHSS was reported (see section 4.4). In rare instances, adnexal torsion (a complication of ovarian enlargement), and haemoperitoneum have been associated with human menopausal gonadotropin therapy. Although these adverse reactions were not observed, there is the possibility that they may also occur with Luveris. Ectopic pregnancy may also occur, especially in women with a history of prior tubal disease. List of adverse reactions The following definitions apply to the frequency terminology used hereafter: very common (≥1/10), common (≥1/100 to <1/10), uncommon (≥1/1,000 to <1/100), rare (≥1/10,000 to <1/1,000), very rare (<1/10,000), frequency not known (cannot be estimated from the available data). The following adverse reactions may be observed after administration of Luveris. Immune system disorders Very rare: Mild to severe hypersensitivity reactions including anaphylactic reactions and shock Nervous system disorders Common: Headache Vascular disorders Very rare: Thromboembolism, usually associated with severe OHSS Gastrointestinal disorders Common: Abdominal pain, abdominal discomfort, nausea, vomiting, diarrhoea Reproductive system and breast disorders Common: Mild or moderate OHSS (including associated symptomatology), ovarian cyst, breast pain, pelvic pain General disorders and administration site conditions: Common: Injection site reaction (e.g. pain, erythema, haematoma, swelling and/or irritation at the site of injection) Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form https://sideeffects.health.gov.il/

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
